Dalton's Law of Partial Pressure
Dalton's Law of Partial Pressure: Overview
This topic covers concepts, such as, Dalton's Law of Partial Pressures of Gases, Derivation of Dalton's Law, Applications of Vapour Pressure, Relative Humidity & Application of Relative Humidity etc.
Important Questions on Dalton's Law of Partial Pressure
A gaseous mixture was prepared by taking equal mole of If the total pressure of the mixture was found 1 atmosphere, the partial pressure of the nitrogen in the mixture is:
If 500ml of gas A at 400 torrs and 666.6 ml of B at 600 torrs are placed in a 3 liter flask, the pressure of the system will be
If 500ml of gas A at 400 torr and 666.6 ml of B at 600 torr are placed in a 3 litre flask, the pressure of the system will be
A mixture of and are trapped in a glass apparatus with a volume of . The pressure of total mixture was of Hg at . The sample was transferred to a bulb in contact with dry ice() so that are frozen out. When the sample returned to the normal value of temperature, the pressure was of Hg. The sample was then transferred to bulb in contact with liquid to freeze out . In the measured volume, the pressure was of Hg at original temperature. Mole of in mixture are .
So, the value of is......(as nearest integer)
A gaseous mixture of moles of , moles of , moles of and moles of is contained in a vessel. Assuming that gases are ideal and the partial pressure of is atm, total pressure is
Which one is not correct mathematical equation for Dalton's Law of partial pressure? Here total pressure of gaseous mixture
A gaseous mixture was prepared by taking equal number of moles of Helium and Neon. If the total pressure of the mixture was found to be the partial pressure of Helium in the mixture is
each of , and are present in a container exerting pressure at . The pressure in atm exerted by each of and in the second container of same volume and temperature is
A mixture of nitrogen and water vapour is admitted to a flask that contains a solid drying agent. Immediately after admission, the pressure in the flask is . After standing for some hours, the pressure reaches a steady value of . Calculate the mole percent of water vapour in the original mixture.
A mixture of and gases in a cylinder contains of and of . If the total pressure of the mixture of the gases in the cylinder is bar, the partial pressure of is:
[Use atomic masses (in ): ]
Dehumidifier is used to reduce the humidity of air. In the following figure a dehumidifier is shown.
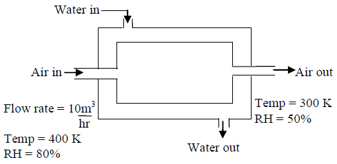
At , saturation vapour pressure .
At , saturation vapour pressure .
If the above dehumidifier is operated for hour how many gram of water will be collected from dehumidifier in nearest possible integer?
A mixture of helium and methane gases at bar pressure contains by mole of helium. The partial
pressure of helium will be :
The ratio of molar masses of ideal gases A and is If pressure of a mixture containing equal mass of
and is , then the partial pressure of will be
Calculate partial pressure (in bar) of , if the density of a mixture of and is at STP.
A mixture contains equal masses of gas at a total pressure of torr. The partial pressure of is
Total pressure is and equal masses of , and are mixed in an empty container at . Calculate the partial pressure of in the mixture. (Assume non reacting mixture)
The total pressure of sample of air containing only and saturated with water vapours is 640 torr. The aqueous tension of water is 40 torr and the molar ratio of is 3 : 1. The partial pressure of in the sample is? (Assume there is no reaction taking place between nitrogen and hydrogen).
In the reaction, initially, we take of and of exerting a total pressure of at a given temperature in a closed vessel. When of is converted into , the partial pressure of would be?
In which of the following mixtures, Dalton's law of partial pressure is not applicable? (Assume room temperature)
The total pressure of a mixture of and is 1.00 bar. Water, which is formed due to reaction between and , is completely removed in the form of liquid to leave pure stains of at a pressure of 0.35 bar. Assuming ideal gas behavior and all pressure measurements were made under the same conditions of temperature and volume, what is the mole fraction of in the original mixture?
